The field of health research is filled with innovative new technologies that aim to benefit patients and society. But many of these advancements, like biomarker tests, medications, and diagnostic tools, don’t end up being adopted by health care systems, not because they’re not effective, but because they are too expensive for the value they provide. Unfortunately, decisions about whether to adopt a new technology are often made after research and development is complete.
Health technology assessment (HTA) is a common way for provincial ministries of health or federal organizations, like the Canadian Agency for Drugs and Technologies in Health (CADTH), to assess a new technology that has already been deemed effective and safe to understand if it is suitable for funding and use in the public health care system. Early HTA aims to assess the cost-effectiveness, likely impact, and potential value of a technology early in the development stage to patients, providers, and payers. The overall aim of early HTA is to channel investment of time and resources into high-value medical products and services. An important feature of early HTA is that it’s relatively inexpensive and quick to complete, but provides potentially significant benefits to a project.
“Early HTA brings that traditional aspect of HTA, as the name implies, earlier into the process, particularly during the product development stages,” said Alex Tam, Research Project Manager for the Health Economics and Decision Sciences teams at Advancing Health. “Many of the techniques used in an early HTA are shared with traditional HTA, but with a greater focus on capturing uncertainty and supporting decisions about whether it makes sense to continue to identify and develop a product.”
“…early HTA could have signalled this issue long before so much time and funding was put into development.”
There is a broad spectrum of techniques used in HTA including cost-effectiveness analysis, meta-analysis, systematic review, and epidemiology.
“When the government declines to fund an effective technology because it’s not cost-effective, it’s really a missed opportunity because an early HTA could have signalled this issue long before so much time and funding was put into development,” said Dr. Nick Bansback, Program Head for Decision Sciences at Advancing Health and Professor at the School of Population and Public Health at UBC.
“This is something that is already commonly done in-house by large pharmaceutical companies early on in the drug development process. But devices, digital technologies, and academic-led research is just starting to catch up. We are now helping other researchers apply this approach to innovations like genomics, digital technologies, and biomarkers.”
Modelling uncertainty
In their presentations and publications about this topic, Tam and Dr. Bansback discuss the concept of decision uncertainty as it relates to the initiation of an HTA. The earlier in the development process, the less is known about a product, including its production cost, accuracy, and other characteristics. This uncertainty means that the HTA can provide more insights about a product and let the researchers know what characteristics they need to target — what is usually referred to as the “target product profile”.
The pair are currently assessing several potential biomarkers that could be combined and turned into a test panel for psoriatic arthritis.
“We’re modelling different sensitivity, specificity, and price to essentially mimic the performance of each combination of biomarkers to see which would be considered cost effective,” explained Tam. “Because each biomarker has a different cost, having this extra information while still in the clinical validation phase helps the developers pick which set of biomarkers to focus on and understand how well the test needs to perform to be viable for the public health system.”
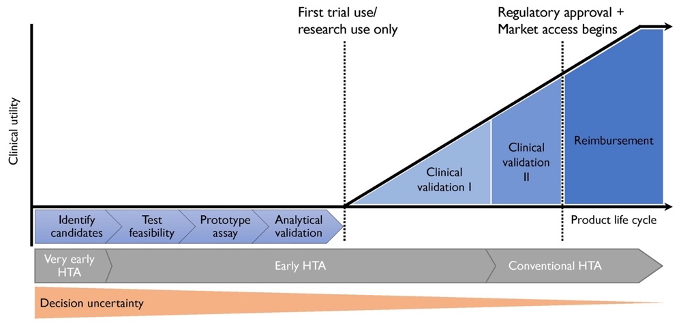
The earlier in the development process, the less is known about a product, including its production cost, accuracy, and other characteristics. This uncertainty means that the HTA can provide more insights about a product and let the researchers know what characteristics they need to target.
Once certain characteristics of a test or technology are determined, there are fewer things to inform about a product. For example, a project led by Advancing Health scientists Drs. Wei Zhang, Annalijn Conklin, and Larry Lynd, with Tam as a co-author, assessed the cost-effectiveness of prostate cancer screening methods. But because the accuracy of the available tests was already determined, the team could only inform potential screening strategies involving the available tests and related price points.
“HTA is applicable across the life cycle of a product,” said Tam. “Early HTA can be applied across the spectrum of development before a product is finalized. What changes is just the amount of information that is available to the modeler.”
Value is relative
Dr. Larry Lynd, Advancing Health scientist and Dean pro tem of the Faculty of Pharmaceutical Sciences at UBC, says that there are numerous reasons that a product or technology might not be adopted into clinical care, but the important thing to consider is whether the technology in question offers enough marginal benefit relative to existing alternatives.
Dr. Lynd and others working in the HTA field use the terms clinical and economic headroom to discuss the incremental benefit a new technology might bring.
“If you’re looking at an indication where there’s unmet need and no quality therapeutic alternative, you have lots of clinical headroom to capture with a new technology,” he explained. “This is different from an indication like syphilis, for example, where penicillin is nearly 100% effective, so there’s very little clinical headroom, and it’s also very cheap, so there’s very little economic headroom.”

Clinical and economic headroom can be thought of as “room for improvement”. Can we find a treatment that is more effective than the current options? Can we find a treatment that is less expensive without sacrificing performance?
Since 2021, Dr. Lynd, along with technical lead Dr. Nick Dragojlovic, has led the development of the early HTA platform for the Nanomedicine Innovation Network (NMIN). The Network develops “smart” medicines to treat or cure disease by delivering small molecule drugs to specific disease sites and enabling the clinical use of gene therapies, and develops diagnostics using a range of nanoparticle technologies.
This work, and other very early applications of HTA, aren’t just about making “go/no go” decisions about a technology during early development, but also looking at different ways of applying a technology to make it viable for the market.
“We just finished an assessment for a group in Montreal who were planning on using a technology for lung cancer. Our results weren’t what they had anticipated so we are now exploring the application of their technology to different, more favourable indications,” he said.
Dr. Lynd emphasizes that early HTA is not just about cost-effectiveness. Though potentially very expensive, the potential of technologies like gene therapies to cure disease means that they can have immense clinical benefit to justify the cost. In such cases, early HTA can be useful to alert governments or other payers that they may have to pay more than expected for a desired outcome.
The future of research?
Recently, some large funding bodies have started to ask for HTA at the outset of a project to improve the outcomes of funded projects. In other cases, Dr. Bansback notes that projects that may take several years to produce final results can use early HTA publications to reassure their funders that the work continues to be a valuable investment.
Early HTA offers researchers with an opportunity to engage with stakeholders, including health care providers, payers, investors, and regulatory agencies, at an early stage. This engagement facilitates a collaborative approach to address the identified challenges and incorporate relevant feedback into the development process.
When applied during the very early stages of product development, Dr. Lynd suggests that early HTA results can also be used to support applications for further funding. An early HTA also makes the economic evaluation of the final product much quicker.
“In early HTA, we’re essentially making a hypothetical model with some assumptions and extrapolations,” he said. “Once the researchers have more data available, we can turn it around and integrate it into the model that we’ve already developed.”
Dr. Bansback, Tam, and Dr. Lynd are part of a small group of experts conducting early HTAs, which are becoming increasingly important as health spending continues to rise as a percentage of GDP. Close to 40 per cent of provincial and territorial budgets are dedicated to health spending.
“We’re now in a really different health care provision paradigm in terms of the costs of treatments and technologies,” said Dr. Lynd. “This shift has pushed us in the direction of early assessment. Hopefully this is just the start of much broader application of early HTA in the future.”



